Molecular sieve is a kind of aluminosilicate compound with cubic lattice. It is mainly composed of silicon and aluminum connected by oxygen bridges to form a hollow skeleton structure. These pores can adsorb molecules smaller than their diameters into the interior of the cavity, and have preferential adsorption capacity for polar molecules and unsaturated molecules, so they can absorb molecules with different polarity, saturation, molecular size and boiling point. Molecules are separated, that is, they have the function of “sieving” molecules, so they are called molecular sieves.
Molecular sieves have excellent properties such as high adsorption capacity, strong thermal stability, high temperature resistance, and strong selectivity that other adsorbents do not have, making molecular sieves widely used in organic chemical, petrochemical, and various fine chemical industries. Its excellent adsorption performance ( When used as a desiccant, a molecular sieve can absorb up to about 22% of its own weight of water), and it is also an excellent adsorbent for air separation and dehydration, air treatment and purification, and even gas dehydration. It is also increasingly popular in the field of waste gas treatment and purification.
Mainly used in the following industries: natural gas, chemical gas, gas separation, water purification treatment, architectural glass, chemical industry, medicine, fertilizer manufacturers, paint coatings, medical oxygen production, packaging, automobile exhaust treatment, etc.
3A molecular sieve
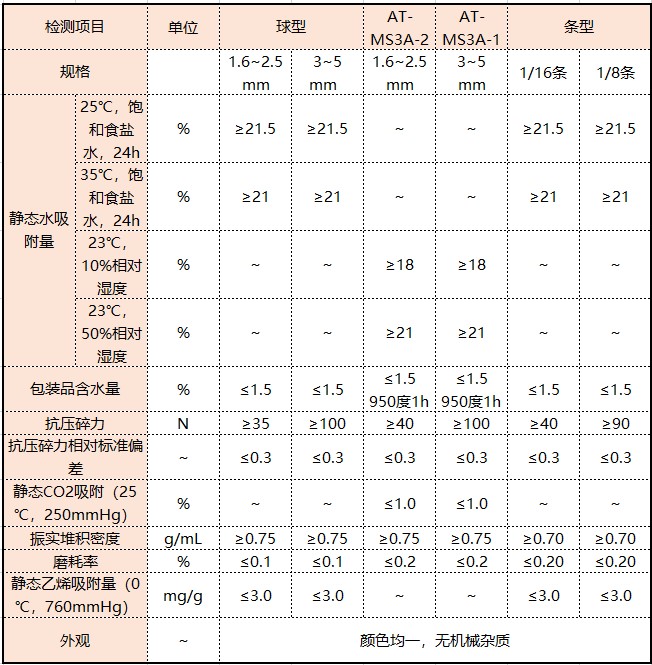
3A molecular sieve: Chemical formula: 2/3K2O·1/3Na2O·Al2O3·2SiO2·9/2H2O English name: 3A Molecular Sieve[1] Silicon-aluminum ratio: SiO2/ Al2O3≈2 Effective pore size: about 0.3nm.
3A molecular sieve refers to potassium A-type aluminosilicate. 3A molecular sieve can adsorb molecules with a critical diameter not greater than 3A. Drying of liquids (such as ethanol, etc.) is a desiccant required for deep drying, refining, and polymerization of gas-liquid phases in petroleum and chemical industries.